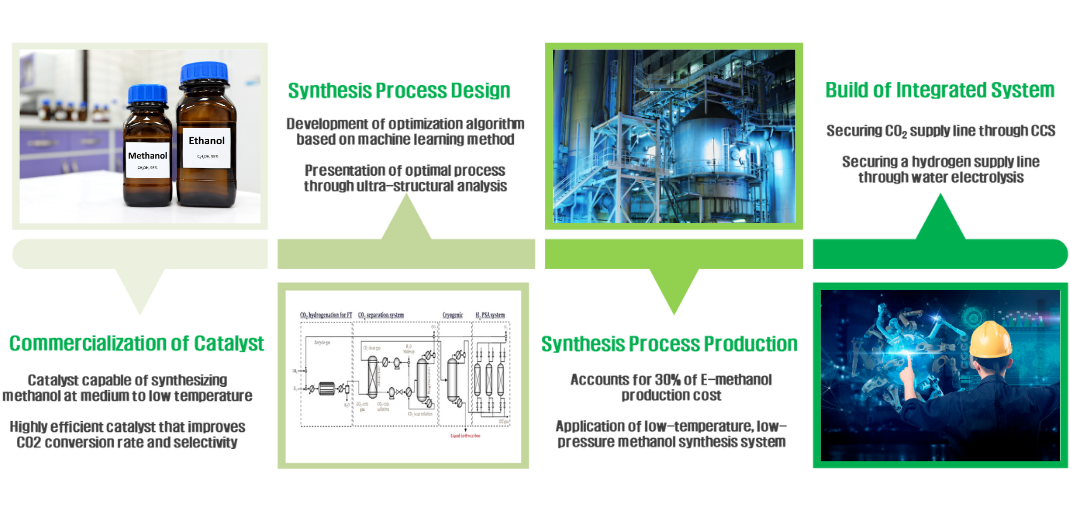
E-methanol, an eco-friendly fuel, is produced through a synthesis reaction of hydrogen produced through water
electrolysis technology using renewable energy and carbon dioxide captured through CCS technology.
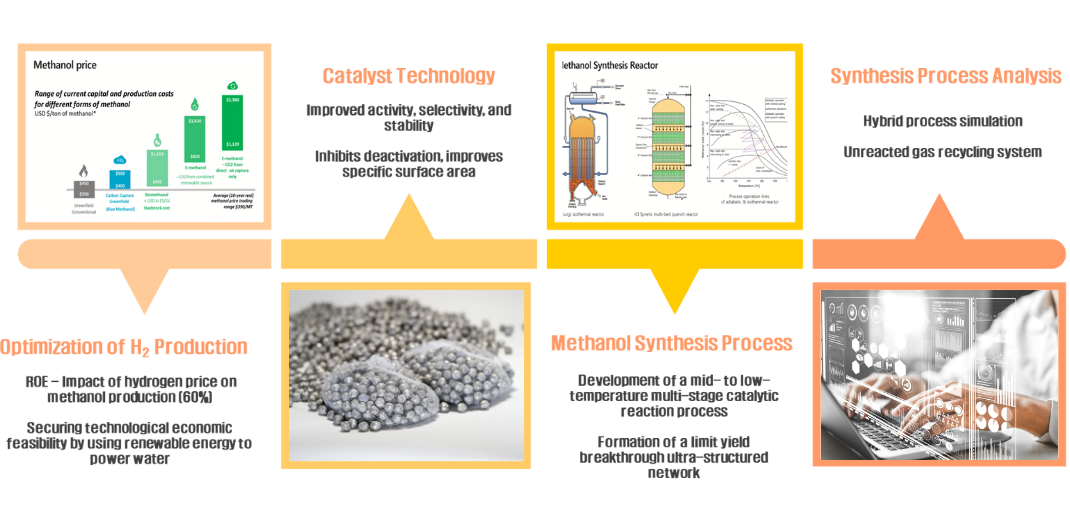
The economics of E-methanol production depend by more than 60% on the cost of hydrogen production.
Additionally, high-temperature and high-pressure synthesis is 30% dependent on energy costs. (820~1,620$/ton
methanol, hydrogen cost 750~1,500$ required)
The mid to low temperature synthesis catalyst improves selectivity, stability, and specific surface area to
produce E-methanol at lower energy. We reduce the unit cost of hydrogen production by improving renewable
energy generation and water electrolysis efficiency, and provide inexpensive, eco-friendly fuel through a
low-temperature, low-pressure methanol manufacturing process.